Using Thalidomide to Treat Cancers such Ovarian Cancer and Plasma Cell Cancer
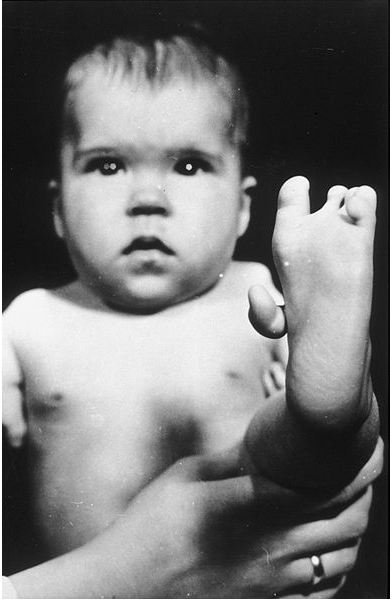
Thalidomide Damages Fetuses
Thalid
omide is a potent teratogenic medication that has the ability to cause damage to fetuses in utero. Women who used thalidomide during their pregnancies back in the 50’s had children with severe birth defects. It was taken to counter the effects of morning sickness as an antiemetic drug for pregnant women. At that time its toxic effects were poorly studied. Its potential toxicity was based erroneously on a structurally similar barbiturate. Years later researchers determined that thalidomide teratogenicity was a result of the drug’s ability to inhibit new blood vessel growth (angiogenesis).
Thalidomide as a Medication for Multiple Melanoma or Plasma Cell Cancer
Multiple myeloma cannot be cured but can be treated. Treatment strategies focus on suppressing and containing out breaks. In 2006, the FDA approved the use of Thalidomide in combination with dexamethasone for treatment against multiple myeloma. Thalidomide has many effects which include inhibiting the growth new blood vessels which feed tumors, inhibits growth of tumor cells, affects the chemical messengers that are involved in myeloma cells and stimulates the immune system to attack cancer cells. Patients use the medication until its effect is maximized. As an added precaution to ensure that pregnant women do not take thalidomide FDA is marketing thalidomide through the System for Thalidomide Education and Prescribing (STEPS) program.
Thalidomide Increases Response in Recurrent Ovarian Cancer
In a recent randomized Phase II clinical trial Thalidomide was found to treat recurring or chronic ovarian cancer. In combination with topotecan an ovarian cancer chemotherapy drug used to treat epithelial ovarian cancer, thalidomide 30 percent of the study’s patients saw their cancer go away. This number is significant when compared to the 18 percent who were only taking topotecan. This study shows the potential of similar thalidomide like compounds to increasing the response of the chemotherapy agents.
Creating a Safer Thalidomide for Cancer Treatment
Besides numerous side effects such as blood clots, seizures and rashes, Thalidomide shows immense potential as an anti-cancer drug. Clinical trials for thalidomide as an anti-cancer drug have shown promising results in cancers such as pancreatic cancer, mantle cell lymphoma and glioma. The drawbacks to the drug are the negative side effects. In response to the side effects, researchers are developing drugs that have Thalidomide like efficacy (analogs of thalidomide) but with lessened side effects. Revlimid (lenalidomide) a derivative of thalidomide has been used to treat multiple melanoma but researchers are still working on ways to prevent the associated side effects.
References
Thalidomide Information. FDA. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107296.htm
Levi Downs, et al, “A prospective randomized trial of thalidomide with topotecan compared with topotecan alone in women with recurrent epithelial ovarian carcinoma” Cancer 2008 Jan 15;112(2):331-9 Abstract
Image Credit
Thalidomide Baby, US Dept. Health